

橢偏儀準在位監(jiān)測薄膜的沉積育苟。利用微腔電解池進行Au基底上薄膜的恒流沉積(-0.4mA)。并在沉積時間為180s椎木、360s违柏、540s、900s香椎、1080s時進行橢偏儀全譜表征(300-800nm)漱竖。
展示全部 
橢偏儀在位表征電化學沉積的系統(tǒng)搭建(六)- 在位監(jiān)測電化學沉積
2.3在位監(jiān)測電化學沉積
目前報道過的在位監(jiān)測手段主要有電化學在位拉曼光譜法畜伐、在位傅里葉紅外光譜儀法闲孤、石英晶振儀法烤礁、質(zhì)譜儀法、在位橢偏儀法肥照。
電化學在位拉曼光譜法脚仔,其原理是通過介質(zhì)分子對入射光發(fā)出頻率的有明顯變化的散射現(xiàn)象,用單色入射光(圓偏振光與線偏振光)來激發(fā)由電極電位控制的電極表面舆绎,然后測定出散射得到的光譜信號鲤脏,如頻率吕朵、強度及偏振性能變化與電極的電位或者電流強度的變化關(guān)系猎醇。
在位傅里葉紅外光譜儀法(FTIRS)是由Bewick等人在20世紀80年代早期首創(chuàng)的努溃。在位傅里葉變換紅外光譜儀可以獲取電極上中性和離子吸附物的分子信息硫嘶,以及參與電化學反應的溶液種類。大量的研究已將在位FTIRS由光滑的表面向粗糙的表面擴展梧税,由靜態(tài)條件向動態(tài)條件擴展沦疾,由水相系統(tǒng)向非水相系統(tǒng)擴展称近。利用在位FTIRS技術(shù)可以得到的電化學雙分子層等圖像信息,達到對電催化反應以及帶電界面過程更深刻的理解哮塞。
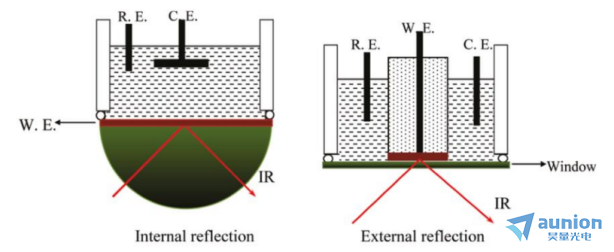
圖1-11兩種在位FTIRS電池設(shè)計圖
兩種在位FTIRS電池設(shè)計方法已經(jīng)被開發(fā)出來刨秆,以減少電解質(zhì)的強紅外吸收,即內(nèi)部和外部反射裝置,其原理圖如圖1-11所示家凯。該方法適用于多種電極材料缓醋,包括金屬單晶電極、納米材料電極肆饶、氧化物材料電極和碳材料電極改衩,并能同時測定電化學反應中吸附物和溶液的種類。在位FTIRS也采用衰減全反射(ATR)模式的內(nèi)反射結(jié)構(gòu)驯镊,在高折射率的紅外透明母棱鏡上沉積一層金屬薄膜作為工作電極葫督。由于紅外光束從電極背面(通過棱鏡)聚焦在界面上,然后檢測到反射輻射板惑,因此溶液層的厚度對入射橄镜、出射光的影響可避免,故而液層的厚度將不再受到限制冯乘。然而洽胶,這種內(nèi)部反射結(jié)構(gòu)的電極材料僅限于紅外窗口棱鏡上的一個薄膜(小于100nm),僅限于濺射或化學沉積的少數(shù)金屬(Au裆馒、Pt姊氓、Pd等)。
石英晶振儀是一種非常靈敏的質(zhì)量天平喷好,可以測量單位面積內(nèi)質(zhì)量的毫微克水平變化。石英是一種壓電材料梗搅,通常通過金屬電極施加適當?shù)碾妷汉萄洌梢允蛊湟砸?guī)定的頻率振蕩。在電極表面添加或去除少量的質(zhì)量可以影響振蕩的頻率无切。這種頻率的變化可以實時監(jiān)測,以獲得電極表面發(fā)生的分子相互作用或反應的有用信息哆键,如薄膜生長掘托、氧化、腐蝕或衰減等籍嘹。因此可以把石英晶振儀作為工作電極襯底烫映,從而用于監(jiān)控薄膜生長過程中的薄膜厚度沼本。石英晶振儀能給出沉積的量的多少,但是無法給出生長的模式锭沟,因此通常用于配合其他的測試方法抽兆,如橢偏儀。
質(zhì)譜儀法是通過用電場族淮、磁場把運動的帶電荷原子辫红、分子和離子等粒子,按其比荷進行分離檢測的方法祝辣。不同帶電粒子其質(zhì)荷比不同,偏轉(zhuǎn)的時間也不同蝙斜,質(zhì)譜儀就可以將這些不同的時間名惩、位置等信息轉(zhuǎn)變成光學數(shù)據(jù),通過質(zhì)譜圖呈現(xiàn)出來孕荠,這樣混合物中的各種成分就可以被解析觀察娩鹉。可以用于解構(gòu)在電化學過程中溶液的變化等稚伍。
了解更多橢偏儀詳情弯予,請訪問上海昊量光電的官方網(wǎng)頁:
http://www.wjjzl.com/three-level-56.html
更多詳情請聯(lián)系昊量光電/歡迎直接聯(lián)系昊量光電
關(guān)于昊量光電:
上海昊量光電設(shè)備有限公司是光電產(chǎn)品專業(yè)代理商个曙,產(chǎn)品包括各類激光器锈嫩、光電調(diào)制器、光學測量設(shè)備垦搬、光學元件等呼寸,涉及應用涵蓋了材料加工、光通訊猴贰、生物醫(yī)療等舔、科學研究、國防糟趾、量子光學甚牲、生物顯微义郑、物聯(lián)傳感丈钙、激光制造等非驮;可為客戶提供完整的設(shè)備安裝,培訓雏赦,硬件開發(fā)劫笙,軟件開發(fā)芙扎,系統(tǒng)集成等服務(wù)。
您可以通過我們昊量光電的官方網(wǎng)站www.wjjzl.com了解更多的產(chǎn)品信息填大,或直接來電咨詢4006-888-532。
相關(guān)文獻:
[1] WONG H S P, FRANK D J, SOLOMON P M et al. Nanoscale cmos[J]. Proceedings of the IEEE, 1999, 87(4): 537-570.
[2] LOSURDO M, HINGERL K. ellipsometry at the nanoscale[M]. Springer Heidelberg New York Dordrecht London. 2013.
[3] DYRE J C. Universal low-temperature ac conductivity of macroscopically disordered nonmetals[J]. Physical Review B, 1993, 48(17): 12511-12526. DOI:10.1103/PhysRevB.48.12511.
[4] CHEN S, KüHNE P, STANISHEV V et al. On the anomalous optical conductivity dISPersion of electrically conducting polymers: Ultra-wide spectral range ellipsometry combined with a Drude-Lorentz model[J]. Journal of Materials Chemistry C, 2019, 7(15): 4350-4362.
[5] 陳籃允华,周巖. 膜厚度測量的橢偏儀法原理分析[J]. 大學物理實驗, 1999, 12(3): 10-13.
[6] ZAPIEN J A, COLLINS R W, MESSIER R. Multichannel ellipsometer for real time spectroscopy of thin film deposition from 1.5 to 6.5 eV[J]. Review of Scientific Instruments, 2000, 71(9): 3451-3460.
[7] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[8] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[9] YUAN M, YUAN L, HU Z et al. In Situ Spectroscopic Ellipsometry for Thermochromic CsPbI3 Phase Evolution Portfolio[J]. Journal of Physical Chemistry C, 2020, 124(14): 8008-8014.
[10] 焦楊景.橢偏儀在位表征電化學沉積的系統(tǒng)搭建.云南大學說是論文,2022.
[11] CANEPA M, MAIDECCHI G, TOCCAFONDI C et al. Spectroscopic ellipsometry of self assembLED monolayers: Interface effects. the case of phenyl selenide SAMs on gold[J]. Physical Chemistry Chemical Physics, 2013, 15(27): 11559-11565. DOI:10.1039/c3cp51304a.
[12] FUJIWARA H, KONDO M, MATSUDA A. Interface-layer formation in microcrystalline Si:H growth on ZnO substrates studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Journal of Applied Physics, 2003, 93(5): 2400-2409.
[13] FUJIWARA H, TOYOSHIMA Y, KONDO M et al. Interface-layer formation mechanism in (formula presented) thin-film growth studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Physical Review B - Condensed Matter and Materials Physics, 1999, 60(19): 13598-13604.
[14] LEE W K, KO J S. Kinetic model for the simulation of hen egg white lysozyme adsorption at solid/water interface[J]. Korean Journal of Chemical Engineering, 2003, 20(3): 549-553.
[15] STAMATAKI K, PAPADAKIS V, EVEREST M A et al. Monitoring adsorption and sedimentation using evanescent-wave cavity ringdown ellipsometry[J]. Applied Optics, 2013, 52(5): 1086-1093.
[16] VIEGAS D, FERNANDES E, QUEIRóS R et al. Adapting Bobbert-Vlieger model to spectroscopic ellipsometry of gold nanoparticles with bio-organic shells[J]. Biomedical Optics Express, 2017, 8(8): 3538.
[17] ARWIN H. Application of ellipsometry techniques to biological materials[J]. Thin Solid Films, 2011, 519(9): 2589-2592.
[18] ZIMMER A, VEYS-RENAUX D, BROCH L et al. In situ spectroelectrochemical ellipsometry using super continuum white laser: Study of the anodization of magnesium alloy [J]. Journal of Vacuum Science & Technology B, 2019, 37(6): 062911.
[19] ZANGOOIE S, BJORKLUND R, ARWIN H. Water Interaction with Thermally Oxidized Porous Silicon Layers[J]. Journal of The Electrochemical Society, 1997, 144(11): 4027-4035.
[20] KYUNG Y B, LEE S, OH H et al. Determination of the optical functions of various liquids by rotating compensator multichannel spectroscopic ellipsometry[J]. Bulletin of the Korean Chemical Society, 2005, 26(6): 947-951.
[21] OGIEGLO W, VAN DER WERF H, TEMPELMAN K et al. Erratum to ― n-Hexane induced swelling of thin PDMS films under non-equilibrium nanofiltration permeation conditions, resolved by spectroscopic ellipsometry‖ [J. Membr. Sci. 431 (2013), 233-243][J]. Journal of Membrane Science, 2013, 437: 312..
[22] BROCH L, JOHANN L, STEIN N et al. Real time in situ ellipsometric and gravimetric monitoring for electrochemistry experiments[J]. Review of Scientific Instruments, 2007, 78(6).
[23] BISIO F, PRATO M, BARBORINI E et al. Interaction of alkanethiols with nanoporous cluster-assembled Au films[J]. Langmuir, 2011, 27(13): 8371-8376.
[24] 李廣立. 氧化亞銅薄膜的制備及其光電性能研究[D]. 西南交通大學, 2016.
[25] 董金礦. 氧化亞銅薄膜的制備及其光催化性能的研究[D]. 安徽建筑大學, 2014.
[26] 張楨. 氧化亞銅薄膜的電化學制備及其光催化和光電性能的研究[D]. 上海交通大學材料科 學與工程學院, 2013.
[27] DISSERTATION M. Cellulose Derivative and Lanthanide Complex Thin Film Cellulose Derivative and Lanthanide Complex Thin Film[J]. 2017.
[28] NIE J, YU X, HU D et al. Preparation and Properties of Cu2O/TiO2 heterojunction Nanocomposite for Rhodamine B Degradation under visible light[J]. ChemistrySelect, 2020, 5(27): 8118-8128.
[29] STRASSER P, GLIECH M, KUEHL S et al. Electrochemical processes on solid shaped nanoparticles with defined facets[J]. Chemical Society Reviews, 2018, 47(3): 715-735.
[30] XU Z, CHEN Y, ZHANG Z et al. Progress of research on underpotential deposition——I. Theory of underpotential deposition[J]. Wuli Huaxue Xuebao/ Acta Physico - Chimica Sinica, 2015, 31(7): 1219-1230.
[31] PANGAROV n. Thermodynamics of electrochemical phase formation and underpotential metal deposition[J]. Electrochimica Acta, 1983, 28(6): 763-775.
[32] KAYASTH S. ELECTRODEPOSITION STUDIES OF RARE EARTHS[J]. Methods in Geochemistry and Geophysics, 1972, 6(C): 5-13.
[33] KONDO T, TAKAKUSAGI S, UOSAKI K. Stability of underpotentially deposited Ag layers on a Au(1 1 1) surface studied by surface X-ray scattering[J]. Electrochemistry Communications, 2009, 11(4): 804-807.
[34] GASPAROTTO L H S, BORISENKO N, BOCCHI N et al. In situ STM investigation of the lithium underpotential deposition on Au(111) in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide[J]. Physical Chemistry Chemical Physics, 2009, 11(47): 11140-11145.
[35] SARABIA F J, CLIMENT V, FELIU J M. Underpotential deposition of Nickel on platinum single crystal electrodes[J]. Journal of Electroanalytical Chemistry, 2018, 819(V): 391-400.
[36] BARD A J, FAULKNER L R, SWAIN E et al. Fundamentals and Applications[M]. John Wiley & Sons, Inc, 2001.
[37] SCHWEINER F, MAIN J, FELDMAIER M et al. Impact of the valence band structure of Cu2O on excitonic spectra[J]. Physical Review B, 2016, 93(19): 1-16.
[38] XIONG L, HUANG S, YANG X et al. P-Type and n-type Cu2O semiconductor thin films: Controllable preparation by simple solvothermal method and photoelectrochemical properties[J]. Electrochimica Acta, 2011, 56(6): 2735-2739.
[39] KAZIMIERCZUK T, FR?HLICH D, SCHEEL S et al. Giant Rydberg excitons in the copper oxide Cu2O[J]. Nature, 2014, 514(7522): 343-347.
[40] RAEBIGER H, LANY S, ZUNGER A. Origins of the p-type nature and cation deficiency in Cu2 O and related materials[J]. Physical Review B - Condensed Matter and Materials Physics, 2007, 76(4): 1-5.
[41] 舒云. Cu2O薄膜的電化學制備及其光電化學性能的研究[D]. 云南大學物理與天文學院,2019.
展示全部 