

加入透明溶液對基底進行測試是可行的议街,但是溶液厚度會對測量結(jié)果帶來數(shù)值上的上下移動,溶液達(dá)到一定厚度后測試得到的數(shù)據(jù)會趨于穩(wěn)定璧榄。在該波段溶液的存在會帶來數(shù)據(jù)的波動特漩。雖然敞開器皿作為池體很簡單方便,但是它也存在溶液敞開會有溶液紊動骨杂,且存在測試時間長涂身、溶液易被污染等對測試不利的因素,故需要重新設(shè)計其他電解池搓蚪。
展示全部 
橢偏儀在位表征電化學(xué)沉積的系統(tǒng)搭建(十六)- 可行性分析
3.2.4可行性分析
(1)光路可行性分析
如圖3-4所示蛤售,為了保證對電極不影響光路的傳輸,其可活動的范圍為圖中h所示妒潭。如果半圓直徑為50px悍抑,對電極寬25px,上限由電極碰到池體壁決定杜耙,則此時入射光的極限入射角為?1=30°搜骡;下限由入射光的入射角決定,圖中的入射角?2=55°佑女,則電極可調(diào)的極限zui低位置如圖所示记靡。所以在滿足對電極不擋光的情況下,入射光的入射角可調(diào)范圍是30°
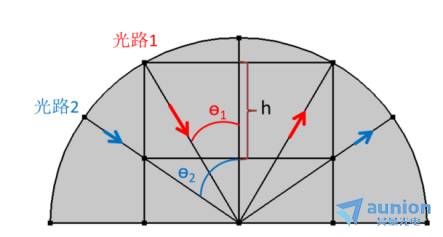
圖3-4觀察窗口光路截面分析圖
用鍍金硅片和電解液(透明溶液)在玻璃皿中調(diào)節(jié)了準(zhǔn)直紊选,不經(jīng)過玻璃皿啼止,溶液中鍍金硅片可以很好的反光;后又把玻璃皿的蓋子蓋上兵罢,驗證得知献烦,在垂直于玻璃蓋、空氣卖词、溶液界面入射時巩那,光斑可以很好的打到電極片上,基本不受光路影響,斜射時光斑就散了即横。經(jīng)過實際驗證噪生,該池體設(shè)計方案可行。
關(guān)于調(diào)節(jié)光斑使其達(dá)到圓心东囚,這也可以實現(xiàn)的杠园,因為一旦不是垂直于池體入射,光斑就是分散的舔庶。
(2)電極的選擇
如圖3-5抛蚁,用Comsol對如圖放置的長正方形電極和圓電極進行了陽極電流密度的模擬結(jié)果如圖3-6示。
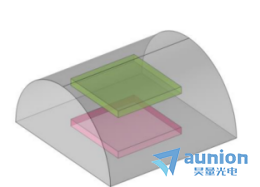
圖3-5電極電流密度模擬圖
從圖3-6中可以看出邊緣效應(yīng)隨著電極的增大而減小惕橙,考慮到電解池尺寸瞧甩,則選則25px×25px的電極片是比較合適的。從c弥鹦、d對比可知肚逸,圓盤電極的邊緣化效應(yīng)比長方形電極邊緣化效應(yīng)更嚴(yán)重,所以選片電極更合適彬坏。
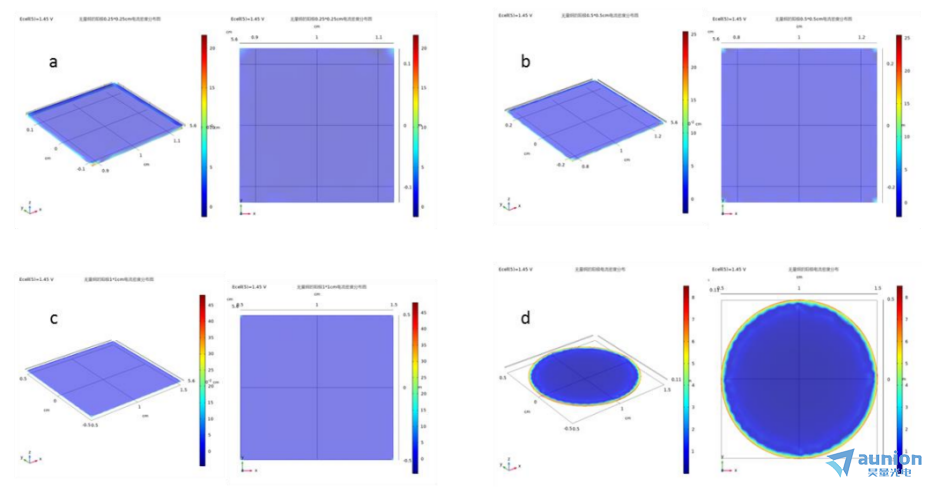
圖3-6不同尺寸電極無量綱電流密度模擬圖(a)6.25px×6.25px朦促;(b)12.5px×12.5px;(c)25px×25px栓始;(d)半徑為12.5px的圓盤電極
3.2.5池體制作
現(xiàn)已經(jīng)完成了制作务冕,如圖3-7所示。池體兩端的長方體及電極載體是亞克力板制作幻赚,中間的半圓柱體由石英玻璃制作禀忆,以上部件定制完成,后期拼接自主完成落恼。

圖3-7電解池實物圖
了解更多橢偏儀詳情箩退,請訪問上海昊量光電的官方網(wǎng)頁:
http://www.wjjzl.com/three-level-56.html
更多詳情請聯(lián)系昊量光電/歡迎直接聯(lián)系昊量光電
關(guān)于昊量光電:
上海昊量光電設(shè)備有限公司是光電產(chǎn)品專業(yè)代理商,產(chǎn)品包括各類激光器佳谦、光電調(diào)制器戴涝、光學(xué)測量設(shè)備、光學(xué)元件等钻蔑,涉及應(yīng)用涵蓋了材料加工啥刻、光通訊、生物醫(yī)療矢棚、科學(xué)研究郑什、國防府喳、量子光學(xué)蒲肋、生物顯微、物聯(lián)傳感、激光制造等兜粘;可為客戶提供完整的設(shè)備安裝申窘,培訓(xùn),硬件開發(fā)孔轴,軟件開發(fā)剃法,系統(tǒng)集成等服務(wù)。
您可以通過我們昊量光電的官方網(wǎng)站www.wjjzl.com了解更多的產(chǎn)品信息路鹰,或直接來電咨詢4006-888-532贷洲。
相關(guān)文獻(xiàn):
[1] WONG H S P, FRANK D J, SOLOMON P M et al. Nanoscale cmos[J]. Proceedings of the IEEE, 1999, 87(4): 537-570.
[2] LOSURDO M, HINGERL K. ellipsometry at the nanoscale[M]. Springer Heidelberg New York Dordrecht London. 2013.
[3] DYRE J C. Universal low-temperature ac conductivity of macroscopically disordered nonmetals[J]. Physical Review B, 1993, 48(17): 12511-12526. DOI:10.1103/PhysRevB.48.12511.
[4] CHEN S, KüHNE P, STANISHEV V et al. On the anomalous optical conductivity dISPersion of electrically conducting polymers: Ultra-wide spectral range ellipsometry combined with a Drude-Lorentz model[J]. Journal of Materials Chemistry C, 2019, 7(15): 4350-4362.
[5] 陳籃,周巖. 膜厚度測量的橢偏儀法原理分析[J]. 大學(xué)物理實驗, 1999, 12(3): 10-13.
[6] ZAPIEN J A, COLLINS R W, MESSIER R. Multichannel ellipsometer for real time spectroscopy of thin film deposition from 1.5 to 6.5 eV[J]. Review of Scientific Instruments, 2000, 71(9): 3451-3460.
[7] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[8] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[9] YUAN M, YUAN L, HU Z et al. In Situ Spectroscopic Ellipsometry for Thermochromic CsPbI3 Phase Evolution Portfolio[J]. Journal of Physical Chemistry C, 2020, 124(14): 8008-8014.
[10] 焦楊景.橢偏儀在位表征電化學(xué)沉積的系統(tǒng)搭建.云南大學(xué)說是論文,2022.
[11] CANEPA M, MAIDECCHI G, TOCCAFONDI C et al. Spectroscopic ellipsometry of self assembLED monolayers: Interface effects. the case of phenyl selenide SAMs on gold[J]. Physical Chemistry Chemical Physics, 2013, 15(27): 11559-11565. DOI:10.1039/c3cp51304a.
[12] FUJIWARA H, KONDO M, MATSUDA A. Interface-layer formation in microcrystalline Si:H growth on ZnO substrates studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Journal of Applied Physics, 2003, 93(5): 2400-2409.
[13] FUJIWARA H, TOYOSHIMA Y, KONDO M et al. Interface-layer formation mechanism in (formula presented) thin-film growth studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Physical Review B - Condensed Matter and Materials Physics, 1999, 60(19): 13598-13604.
[14] LEE W K, KO J S. Kinetic model for the simulation of hen egg white lysozyme adsorption at solid/water interface[J]. Korean Journal of Chemical Engineering, 2003, 20(3): 549-553.
[15] STAMATAKI K, PAPADAKIS V, EVEREST M A et al. Monitoring adsorption and sedimentation using evanescent-wave cavity ringdown ellipsometry[J]. Applied Optics, 2013, 52(5): 1086-1093.
[16] VIEGAS D, FERNANDES E, QUEIRóS R et al. Adapting Bobbert-Vlieger model to spectroscopic ellipsometry of gold nanoparticles with bio-organic shells[J]. Biomedical Optics Express, 2017, 8(8): 3538.
[17] ARWIN H. Application of ellipsometry techniques to biological materials[J]. Thin Solid Films, 2011, 519(9): 2589-2592.
[18] ZIMMER A, VEYS-RENAUX D, BROCH L et al. In situ spectroelectrochemical ellipsometry using super continuum white laser: Study of the anodization of magnesium alloy [J]. Journal of Vacuum Science & Technology B, 2019, 37(6): 062911.
[19] ZANGOOIE S, BJORKLUND R, ARWIN H. Water Interaction with Thermally Oxidized Porous Silicon Layers[J]. Journal of The Electrochemical Society, 1997, 144(11): 4027-4035.
[20] KYUNG Y B, LEE S, OH H et al. Determination of the optical functions of various liquids by rotating compensator multichannel spectroscopic ellipsometry[J]. Bulletin of the Korean Chemical Society, 2005, 26(6): 947-951.
[21] OGIEGLO W, VAN DER WERF H, TEMPELMAN K et al. Erratum to ― n-Hexane induced swelling of thin PDMS films under non-equilibrium nanofiltration permeation conditions, resolved by spectroscopic ellipsometry‖ [J. Membr. Sci. 431 (2013), 233-243][J]. Journal of Membrane Science, 2013, 437: 312..
[22] BROCH L, JOHANN L, STEIN N et al. Real time in situ ellipsometric and gravimetric monitoring for electrochemistry experiments[J]. Review of Scientific Instruments, 2007, 78(6).
[23] BISIO F, PRATO M, BARBORINI E et al. Interaction of alkanethiols with nanoporous cluster-assembled Au films[J]. Langmuir, 2011, 27(13): 8371-8376.
[24] 李廣立. 氧化亞銅薄膜的制備及其光電性能研究[D]. 西南交通大學(xué), 2016.
[25] 董金礦. 氧化亞銅薄膜的制備及其光催化性能的研究[D]. 安徽建筑大學(xué), 2014.
[26] 張楨. 氧化亞銅薄膜的電化學(xué)制備及其光催化和光電性能的研究[D]. 上海交通大學(xué)材料科 學(xué)與工程學(xué)院, 2013.
[27] DISSERTATION M. Cellulose Derivative and Lanthanide Complex Thin Film Cellulose Derivative and Lanthanide Complex Thin Film[J]. 2017.
[28] NIE J, YU X, HU D et al. Preparation and Properties of Cu2O/TiO2 heterojunction Nanocomposite for Rhodamine B Degradation under visible light[J]. ChemistrySelect, 2020, 5(27): 8118-8128.
[29] STRASSER P, GLIECH M, KUEHL S et al. Electrochemical processes on solid shaped nanoparticles with defined facets[J]. Chemical Society Reviews, 2018, 47(3): 715-735.
[30] XU Z, CHEN Y, ZHANG Z et al. Progress of research on underpotential deposition——I. Theory of underpotential deposition[J]. Wuli Huaxue Xuebao/ Acta Physico - Chimica Sinica, 2015, 31(7): 1219-1230.
[31] PANGAROV n. Thermodynamics of electrochemical phase formation and underpotential metal deposition[J]. Electrochimica Acta, 1983, 28(6): 763-775.
[32] KAYASTH S. ELECTRODEPOSITION STUDIES OF RARE EARTHS[J]. Methods in Geochemistry and Geophysics, 1972, 6(C): 5-13.
[33] KONDO T, TAKAKUSAGI S, UOSAKI K. Stability of underpotentially deposited Ag layers on a Au(1 1 1) surface studied by surface X-ray scattering[J]. Electrochemistry Communications, 2009, 11(4): 804-807.
[34] GASPAROTTO L H S, BORISENKO N, BOCCHI N et al. In situ STM investigation of the lithium underpotential deposition on Au(111) in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide[J]. Physical Chemistry Chemical Physics, 2009, 11(47): 11140-11145.
[35] SARABIA F J, CLIMENT V, FELIU J M. Underpotential deposition of Nickel on platinum single crystal electrodes[J]. Journal of Electroanalytical Chemistry, 2018, 819(V): 391-400.
[36] BARD A J, FAULKNER L R, SWAIN E et al. Fundamentals and Applications[M]. John Wiley & Sons, Inc, 2001.
[37] SCHWEINER F, MAIN J, FELDMAIER M et al. Impact of the valence band structure of Cu2O on excitonic spectra[J]. Physical Review B, 2016, 93(19): 1-16.
[38] XIONG L, HUANG S, YANG X et al. P-Type and n-type Cu2O semiconductor thin films: Controllable preparation by simple solvothermal method and photoelectrochemical properties[J]. Electrochimica Acta, 2011, 56(6): 2735-2739.
[39] KAZIMIERCZUK T, FR?HLICH D, SCHEEL S et al. Giant Rydberg excitons in the copper oxide Cu2O[J]. Nature, 2014, 514(7522): 343-347.
[40] RAEBIGER H, LANY S, ZUNGER A. Origins of the p-type nature and cation deficiency in Cu2 O and related materials[J]. Physical Review B - Condensed Matter and Materials Physics, 2007, 76(4): 1-5.
[41] 舒云. Cu2O薄膜的電化學(xué)制備及其光電化學(xué)性能的研究[D]. 云南大學(xué)物理與天文學(xué)院晋柱,2019.
展示全部 